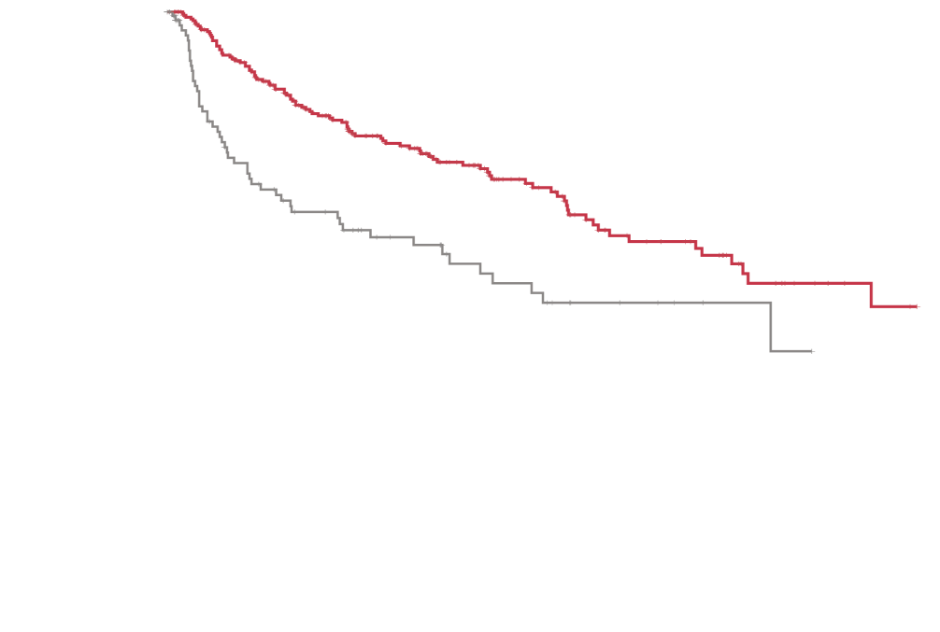
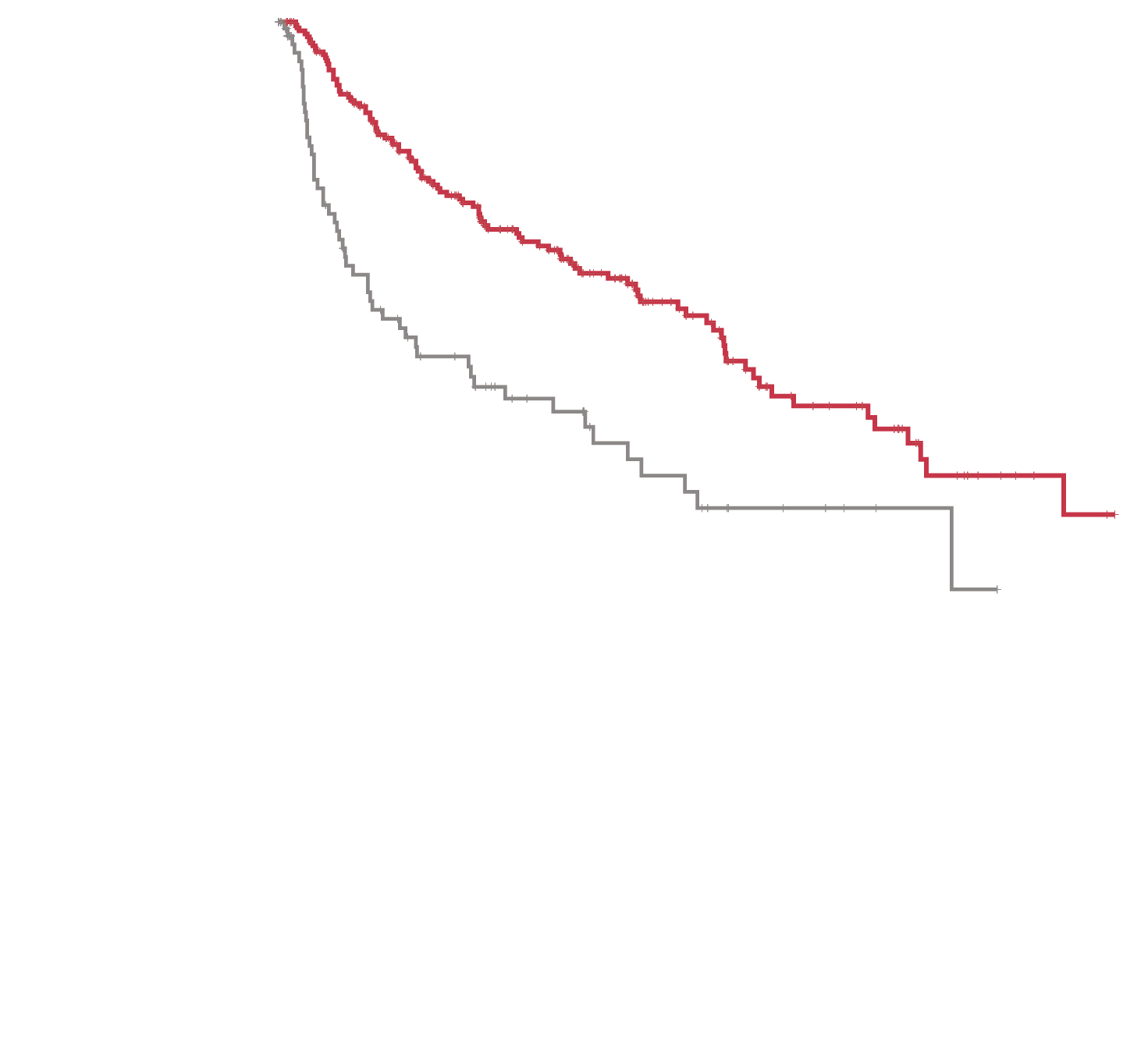
AML=acute myeloid leukemia; FLT3=FMS-like tyrosine kinase 3; ITD=internal tandem duplication.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia V.2.2022. © National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed 06-30-2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366(12):1079-89. 3. Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia 2011;25(6):939-44. 4. Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 2012;485(7397):260-3. 5. Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008;111(5):2776-84. 6. McCormick SR, McCormick MJ, Grutkoski PS, et al. FLT3 mutations at diagnosis and relapse in acute myeloid leukemia: cytogenetic and pathologic correlations, including cuplike blast morphology. Arch Pathol Lab Med 2010;134(8):1143-51. 7. Leisch M, Jansko B, Zaborsky N, Greil R, Pleyer L. Next generation sequencing in AML—on the way to becoming a new standard for treatment initiation and/or modulation? Cancers (Basel) (Epub) 02-21-2019. 8. Morita K, Wang F, Jahn K, et al. Clonal evolution of acute myeloid leukemia revealed by high-throughput single-cell genomics. Nature Commun 2020;11:5327. Erratum in: Nat Commun 2020;11(1):5996. Erratum in: Nat Commun 2021;12(1):2823. 9. Patnaik MM. The importance of FLT3 mutational analysis in acute myeloid leukemia. Leuk Lymphoma 2018;59(10):2273-86. 10. Invivoscribe. LeukoStrat® CDx FLT3 mutation assay. https://catalog.invivoscribe.com/product/leukostrat-cdx-flt3-mutation-assay-2/. Accessed 10-21-2021. 11. Nazha A, Cortes J, Faderl S, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica 2012;97(8):1242-5. 12. Warren M, Luthra R, Yin CC, et al. Clinical impact of change of FLT3 mutation status in acute myeloid leukemia patients. Mod Pathol 2012;25(10):1405-12. 13. Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: feasibility and preliminary efficacy of the Beat AML Master Trial. Nat Med 2020;26(12):1852-8.